Safety of Lithium-Ion batteries

Lithium-Ion refers to a family of Lithium-based battery technology. This family includes several sub-families or technologies, such as:
- LCO: Lithium Cobalt Oxide
- NCA: Nickel Cobalt Aluminium
- NMC: Nickel Manganese Cobalt
- LiFePO4 or LFP: Lithium Iron Phosphate
- LTO: Lithium Titanate Oxide, etc…
Often, we can hear that a product is equipped with “Lithium-Ion” batteries, this does not really have any meaning on the technology used. However, out of habit, the technology referred to as Lithium_Ion is usually LCO, NCA or NMC
Each of these technologies has very different characteristics, particularly in terms of safety, which can be found in the table below.
| Technology | Pros / Cons | Application field |
|---|---|---|
Lithium Iron Phosphate Solid State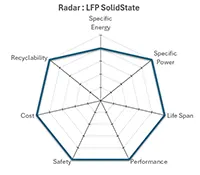 |
|
|
Lithium Iron Phosphate (LFP-LiFePO4)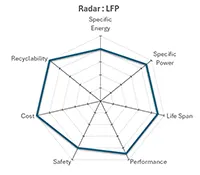 |
|
|
Lithium-Cobalt-Oxyde (LCO)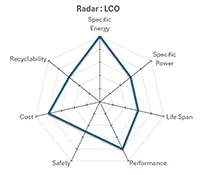 |
|
|
Lithium Nickel Cobalt Aluminium (NCA)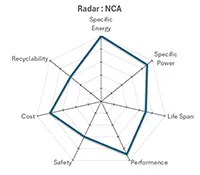 |
|
|
Lithium Nickel Manganese Cobalt (NMC)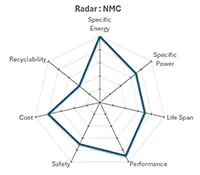 |
|
|
Thermal Runaway
One of the main causes of danger for lithium-ion cells is related to the phenomenon of thermal runaway. This is a heating reaction of the battery in use, caused by the nature of the materials used in the chemistry of the battery.
Thermal runaway is mainly caused by the solicitation of batteries under specific conditions, such as overload under adverse climatic conditions. The result of a thermal runaway of a cell depends on its level of charge and can lead in the worst case to an inflammation or even an explosion of the Lithium-Ion cell.
However, not all types of Lithium-Ion technology, due to their chemical composition, have the same sensitivity to this phenomenon.
The figure below shows the energy produced during an artificially induced thermal runaway
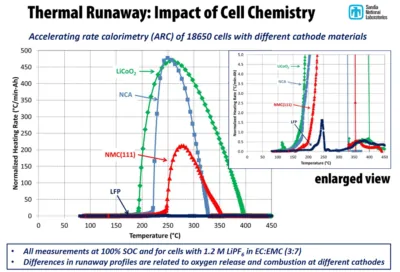
It can be seen that among the Lithium Ion technologies mentioned above, LCO and NCA are the most dangerous chemicals from a thermal runaway point of view with a temperature rise of about 470°C per minute.
The NMC chemistry emits about half the energy, with an increase of 200°C per minute, but this level of energy causes in all cases the internal combustion of materials and the ignition of the cell.
In addition, it can be seen that LiFePO4 – LFP technology is slightly subject to thermal runaway phenomena, with a temperature rise of barely 1.5°C per minute.
With this very low level of energy released, the thermal runaway of the Lithium Iron Phosphate technology is an inherently improbable event in normal operation, and even very difficult to artificially trigger.
More recently, with the arrival of LFP Solid-State technology, the level of safety now exceeds all safety standards, with a thermal runaway that is intrinsically impossible to trigger in normal operation.
Combined with a BMS, Lithium Iron Phosphate (LifePO4 – LFP) is currently the most secure Lithium-Ion technology on the market.
Mecanical Safety of Lithium-Ion Cells
Like thermal runaway, Lithium-ion cells have a different level of safety depending on the shocks or mechanical treatments they may undergo during their lifetime.
The nail penetration test is the most revealing way to qualify level of safety of Lithium-Ion batteries.
The test presented below is performed by perforating a Lithium Ion NMC cell and a Lithium Ion LiFePO4 cell.
We find here the same extremely stable behavior of Lithium Iron Phosphate cells while the NMC cell ignites almost immediately.
For information, the LCO, NCA, or Lithium Polymer cells have a similar behavior to the NMC in a perforation test (immediate inflammation)
Stress Tests of Lithium Chemistries Lithium Polymer (LiPo) vs Lithium Titanate (LTO) vs Lithium Iron Phosphate (LFP) :















 France
France Europe
Europe Rest of the world
Rest of the world